Distillation plants
Separation of liquid mixtures
SCHRADER distillation plants
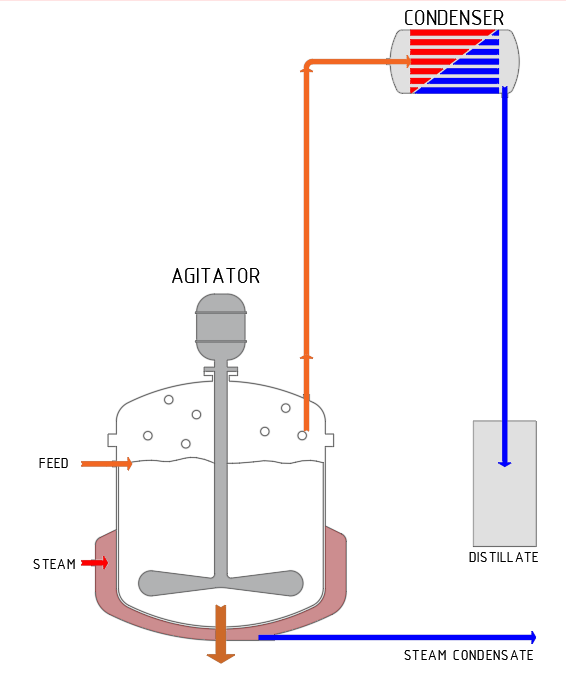
Distillation is a thermal process for the separation of liquid mixtures. The process does not result in complete separation, rather it separates the liquid into two mixtures with predominantly more volatile and predominantly less volatile components. The more volatile components accumulate in the vapour phase and are then separated and condensed (distillate). The predominantly non-volatile components are left behind in the liquid phase.
Distillation has the advantage that there is normally no need to add any additional substances.
SCHRADER distillation plants
are used, for example, in the production of alcohol-based liquid flavours from fruit. Distillation increases the alcohol concentration to the value specified by the customer. The more volatile component is called distillate: this contains the aromatic compounds of the fruits, bound to the alcoholic solvent. This distillate is the product for the customer. The less volatile components remain as an aqueous solution with a low alcohol and aroma content; in most cases, this is disposed of.
Special forms of distillation/rectification
In steam drag distillation, e.g. in the extraction of essential oils, the low-volatility substance which is unsolved in water is carried along by the steam.
In vacuum distillation, the pressure in the distillation plant is reduced, causing the boiling point of the mixture to be separated to decrease. This allows even components that are not sufficiently temperature-stable to be distillated, e.g. natural extracts or highly sensitive raw materials in the pharmaceutical sector.
Contact
Our global network of experienced engineers is available to you by phone or e-mail. Don’t hesitate to get in touch.